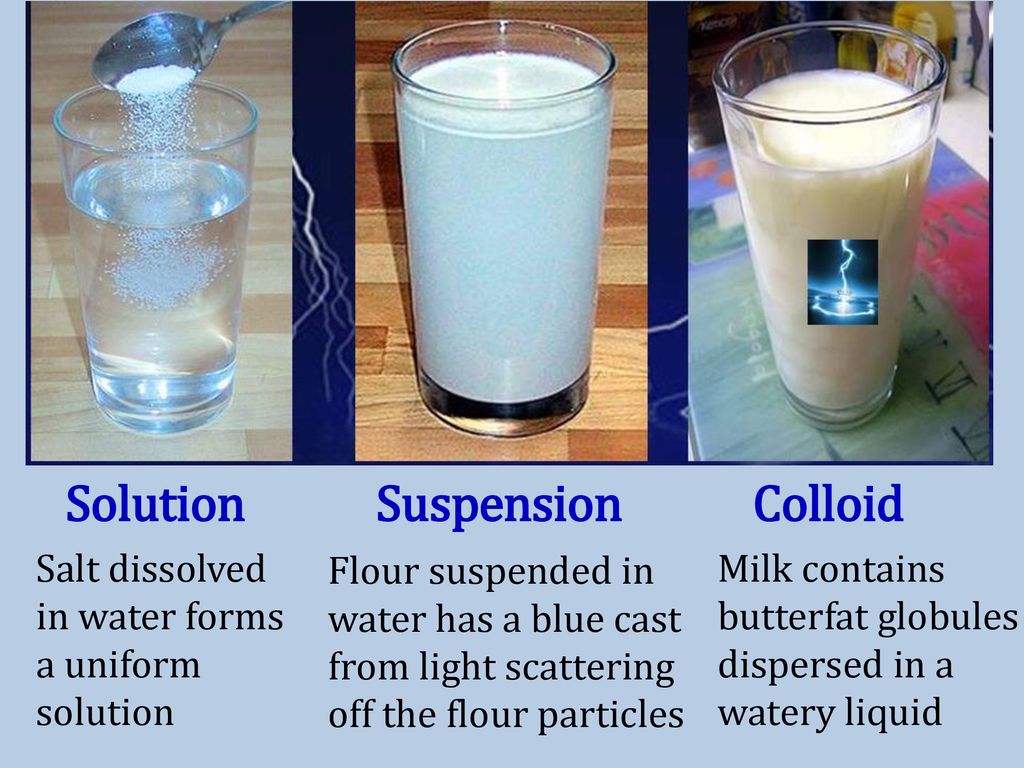
Solution Colloid Suspension Definition . See how it differs from a solution or suspension. a colloid is an intermediate between solution and suspension. The particles are between the sizes of these other two mixtures and the mixture. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. learn what a colloid is in chemistry. a colloid is somewhere in between a solution and a suspension. A colloid is easily visible to. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions. Get the definition and colloid examples. Hydrophilic colloids contain an outer shell of. a colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. It has particles with sizes between 2 and 1000 nanometers. the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium.
from slideplayer.com
a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. The particles are between the sizes of these other two mixtures and the mixture. a colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. Get the definition and colloid examples. learn what a colloid is in chemistry. It has particles with sizes between 2 and 1000 nanometers. a colloid is somewhere in between a solution and a suspension. Hydrophilic colloids contain an outer shell of. See how it differs from a solution or suspension. a colloid is an intermediate between solution and suspension.
Solutions, Colloids, and Suspensions ppt download
Solution Colloid Suspension Definition a colloid is somewhere in between a solution and a suspension. It has particles with sizes between 2 and 1000 nanometers. a colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. The particles are between the sizes of these other two mixtures and the mixture. Get the definition and colloid examples. A colloid is easily visible to. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Hydrophilic colloids contain an outer shell of. See how it differs from a solution or suspension. learn what a colloid is in chemistry. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions. a colloid is an intermediate between solution and suspension. a colloid is somewhere in between a solution and a suspension. the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium.
From chemistnotes.com
Colloids Definition, Examples, Properties, and 10 Reliable Solution Colloid Suspension Definition Hydrophilic colloids contain an outer shell of. a colloid is somewhere in between a solution and a suspension. Get the definition and colloid examples. The particles are between the sizes of these other two mixtures and the mixture. a colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as. Solution Colloid Suspension Definition.
From materialcampuseichel.z19.web.core.windows.net
Solutions Colloids And Suspensions Ppt Solution Colloid Suspension Definition the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. Get the definition and colloid examples. Hydrophilic colloids contain an outer shell of. The particles are between the sizes of these other two mixtures and the mixture. a colloid can be distinguished from a true solution by its. Solution Colloid Suspension Definition.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Solution Colloid Suspension Definition a colloid is somewhere in between a solution and a suspension. learn what a colloid is in chemistry. the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. Hydrophilic colloids contain an outer shell of. The particles are between the sizes of these other two mixtures and. Solution Colloid Suspension Definition.
From www.slideserve.com
PPT Colloids, Solutions, Suspensions PowerPoint Presentation, free Solution Colloid Suspension Definition Hydrophilic colloids contain an outer shell of. Get the definition and colloid examples. a colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. a colloid is somewhere in between a solution and a suspension. It has particles with sizes between 2 and 1000 nanometers. See. Solution Colloid Suspension Definition.
From cekjtznh.blob.core.windows.net
Colloid Compound Solution Suspension at Robert Rutledge blog Solution Colloid Suspension Definition a colloid is somewhere in between a solution and a suspension. Hydrophilic colloids contain an outer shell of. See how it differs from a solution or suspension. the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. The particles are between the sizes of these other two mixtures. Solution Colloid Suspension Definition.
From cekjtznh.blob.core.windows.net
Colloid Compound Solution Suspension at Robert Rutledge blog Solution Colloid Suspension Definition a colloid is somewhere in between a solution and a suspension. the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. Hydrophilic colloids contain an outer shell of. learn what a colloid is in chemistry. Get the definition and colloid examples. a colloid is an intermediate. Solution Colloid Suspension Definition.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Solution Colloid Suspension Definition the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. It has particles with sizes between 2 and 1000 nanometers. a colloid is an intermediate between solution and suspension. a colloid is somewhere in between a solution and a suspension. learn what a colloid is in. Solution Colloid Suspension Definition.
From exouqcyfe.blob.core.windows.net
What Is Colloidal Suspension In Chemistry at Robert Lemond blog Solution Colloid Suspension Definition a colloid is somewhere in between a solution and a suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions. See how it differs from a solution or suspension. Hydrophilic colloids contain an outer shell of. learn what a colloid is in chemistry. a colloid is an. Solution Colloid Suspension Definition.
From www.slideshare.net
Solutions, suspensions, and colloids Solution Colloid Suspension Definition Get the definition and colloid examples. a colloid is an intermediate between solution and suspension. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Hydrophilic colloids contain an outer shell of. It has particles with sizes between 2 and 1000 nanometers. the stability of. Solution Colloid Suspension Definition.
From www.pinterest.com
solutionssuspensionsandcolloids9728.jpg (728×546) Science Solution Colloid Suspension Definition a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. Get the definition and colloid examples. It has particles with sizes between 2 and 1000 nanometers. the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium.. Solution Colloid Suspension Definition.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Solution Colloid Suspension Definition See how it differs from a solution or suspension. a colloid is an intermediate between solution and suspension. a colloid can be distinguished from a true solution by its ability to scatter a beam of light, known as the tyndall effect. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between. Solution Colloid Suspension Definition.
From www.youtube.com
Solution, Colloid or Suspension? The definition in Chemistry and Solution Colloid Suspension Definition Get the definition and colloid examples. See how it differs from a solution or suspension. a colloid is somewhere in between a solution and a suspension. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. The particles are between the sizes of these other two. Solution Colloid Suspension Definition.
From cegexkfz.blob.core.windows.net
Colloid Solutions Quizlet at Katrina Cohen blog Solution Colloid Suspension Definition Get the definition and colloid examples. See how it differs from a solution or suspension. a colloid is an intermediate between solution and suspension. The particles are between the sizes of these other two mixtures and the mixture. a colloid is somewhere in between a solution and a suspension. A colloid is easily visible to. a colloid. Solution Colloid Suspension Definition.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Solution Colloid Suspension Definition It has particles with sizes between 2 and 1000 nanometers. a colloid is an intermediate between solution and suspension. Get the definition and colloid examples. learn what a colloid is in chemistry. Hydrophilic colloids contain an outer shell of. A colloid is easily visible to. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate. Solution Colloid Suspension Definition.
From www.aakash.ac.in
Colloidal Solution Definition, Classification, Examples & Preparation Solution Colloid Suspension Definition Hydrophilic colloids contain an outer shell of. The particles are between the sizes of these other two mixtures and the mixture. a colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a. a colloid is an intermediate between solution and suspension. See how it differs from a. Solution Colloid Suspension Definition.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Solution Colloid Suspension Definition the stability of a colloid system is defined by the measure of particles that remain suspended in solution at equilibrium. learn what a colloid is in chemistry. Get the definition and colloid examples. a colloid is somewhere in between a solution and a suspension. a colloid is an intermediate between solution and suspension. A colloid is. Solution Colloid Suspension Definition.
From www.aquaportail.com
Solution colloïdale définition et explications Solution Colloid Suspension Definition Get the definition and colloid examples. Hydrophilic colloids contain an outer shell of. a colloid is an intermediate between solution and suspension. a group of mixtures called colloids (or colloidal dispersions) exhibit properties intermediate between those of suspensions and solutions. learn what a colloid is in chemistry. It has particles with sizes between 2 and 1000 nanometers.. Solution Colloid Suspension Definition.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Solution Colloid Suspension Definition learn what a colloid is in chemistry. The particles are between the sizes of these other two mixtures and the mixture. A colloid is easily visible to. See how it differs from a solution or suspension. a colloid is an intermediate between solution and suspension. Get the definition and colloid examples. a colloid is somewhere in between. Solution Colloid Suspension Definition.